Wavelengths are emitted when electrons change energy levels from a higher to a lower position, and the transition that gives light the shortest wavelength is the transition with the most energy.
There is no set number for that, as there is a new formula for every wavelength of light produced.
Conversely, small amounts of energy results in larger wavelengths. For example, when we are sleeping, our brains are emitting long and slow wavelengths called delta wavelengths.
While awake, our brains are very fast and moving at gamma and beta speed. Quantum physics and light energy wavelengths function the same way.
The transition that gives light the shortest wavelength is the one that produces the most energy change.
There is no one answer, such as n = 3 – how energy changes are quantified – for what transition is the shortest wavelength.
The electrons that produce or emit this energy change levels in a manner that is like going downstairs.
Table of Contents
What is the relationship between electrons and wavelengths?
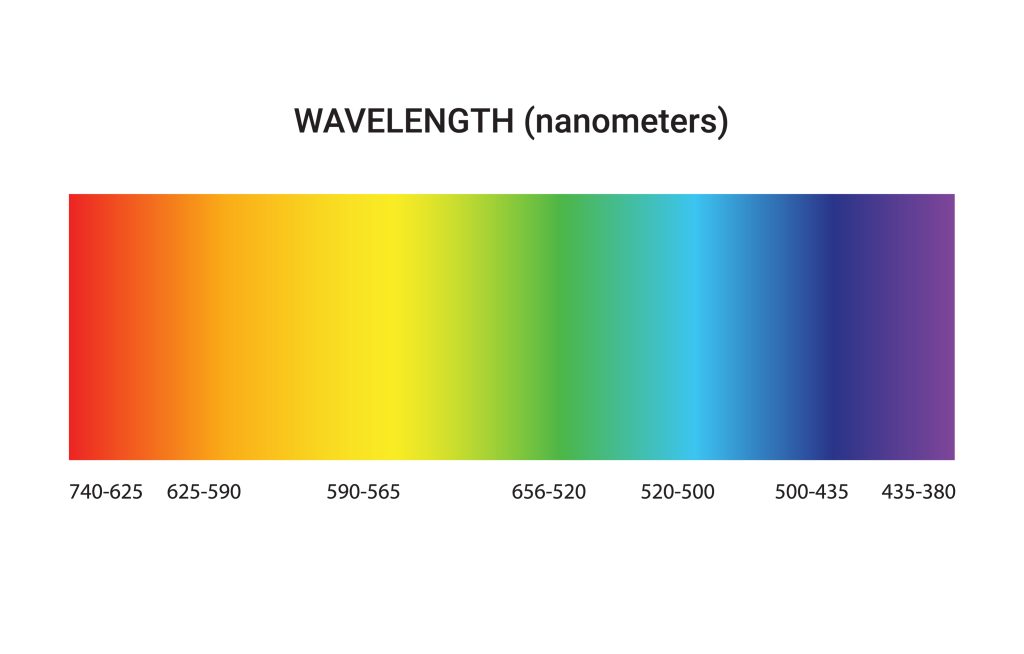
The electron is what makes the transition when calculating the wavelengths of light. The electron that goes the farthest when transitioning between wavelengths, from longer wavelengths to shorter ones, like going down one stair at a time, will be the electron that emits the most energy.
If it were a set of stairs, the electron that goes the farthest will be considered the electron that has the most energy.
When there is movement between energy levels, particularly one from a higher level to a lower level, the movement is called emission.
That is an emission of energy. So, you’ll see fuel wastes emitted from a vehicle’s muffler, and this is an emission of energy that goes from a higher level to a lower level of energy.
Electrons are the components of quantum physics that emit energy which in some cases becomes light.
In order to see the wavelength in a way that we can call it light, it needs to transition upwards in energy levels.
When it does, the wavelengths shorten, and you will see “n” values increase.
What is the “n” in light and wavelength theory?
The “n” is a value in quantum physics that is also called the principal quantum number. There are four quantum numbers in total, and each of them will be assigned to an electron in one atom.
This number signifies how much energy an electron will have.
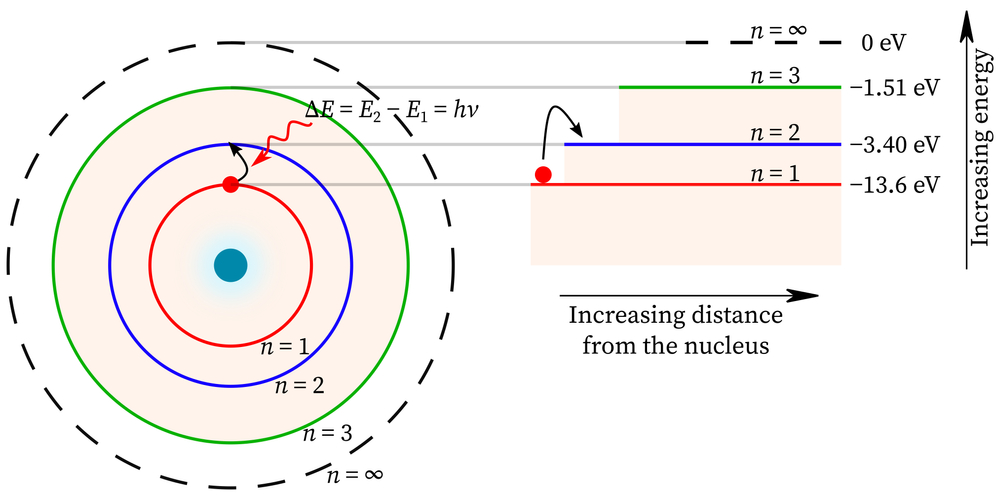
The energy level is a fixed number and signifies how much distance a nucleus is from any atom.
The numbers are whole numbers, such as 1, 2, 3, or 4, and so on. The electron with the lowest number is the electron that is closest to the nucleus.
You will see these numbers on the periodic table, where the principal quantum number, or, n, refers to the row number of an atom.
The atom, in this case, is the largest particle and is comprised of a nucleus in the center that is surrounded by protons, neutrons, and electrons.
The electron is the farthest away and carries the most energy or charge in an atom, although it is a negative charge.
When n increases, the electron becomes more distanced from the nucleus. The elements closest to the nucleus will have the least amount of energy, such as the protons and the neutral neutrons.
The electrons will orbit the nucleus though, and when they do, their energy changes. The energy change is what is called the distance the electron has traveled.
And this is how an electron distance becomes a wavelength.
How are distances of electrons measured?
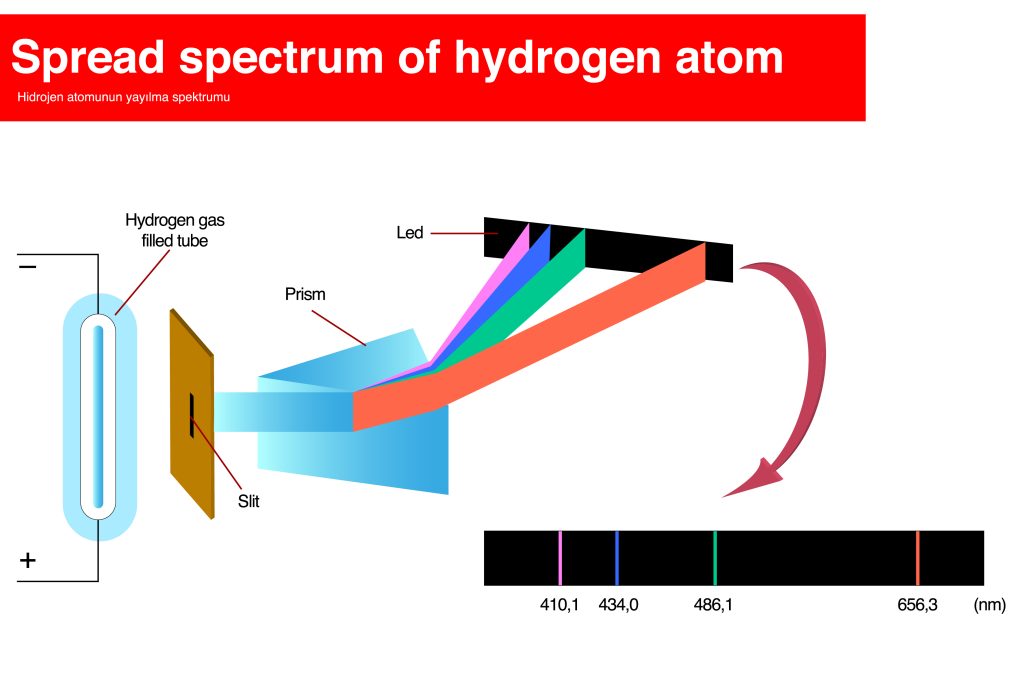
Distances of electrons are measured by energy changes that calculate wavelengths of ultraviolet light along the hydrogen spectrum.
As with everything in science, every distance discovered was discovered by a person. Thus, the distances electrons travel, or their energy changes, are called by the names of those that discovered these miracles.
The Bohr model is the model that illustrates how electrons orbit the nucleus, as discovered by Nobel-prize-winning quantum physics expert Niels Bohr, just prior to his 1922 Nobel win.
The Lyman series signifies when an electron moves from n = 2 to n = 1 – discovered by Theodore Lyman in 1906 before Bohr’s model was discovered – when he wondered how energy changed in the ultraviolet spectrum.
The Balmer series followed, and studied the energy level changes from n = 3 to n = 2, as discovered by Johann Balmer in 1885 who had been studying the hydrogen spectrum.
He used simple math to discover the wavelengths of lines along the hydrogen spectrum. Other series would follow, including the Paschen series, when energy changes from n = 4 to n = 3.
The Brackett series occurs when energy changes from n = 5 to n = 4, and the Pfund series occurs when energy changes from n = 6 to n = 5.
In the Humphrey series, discovered by Curtis Humphreys in 1955, the electrons move much slower and are defined by energy levels changing from n values greater or equal to 7 down to 6.
These values are often found in transitions of infrared light.
How are light wavelength transitions calculated?
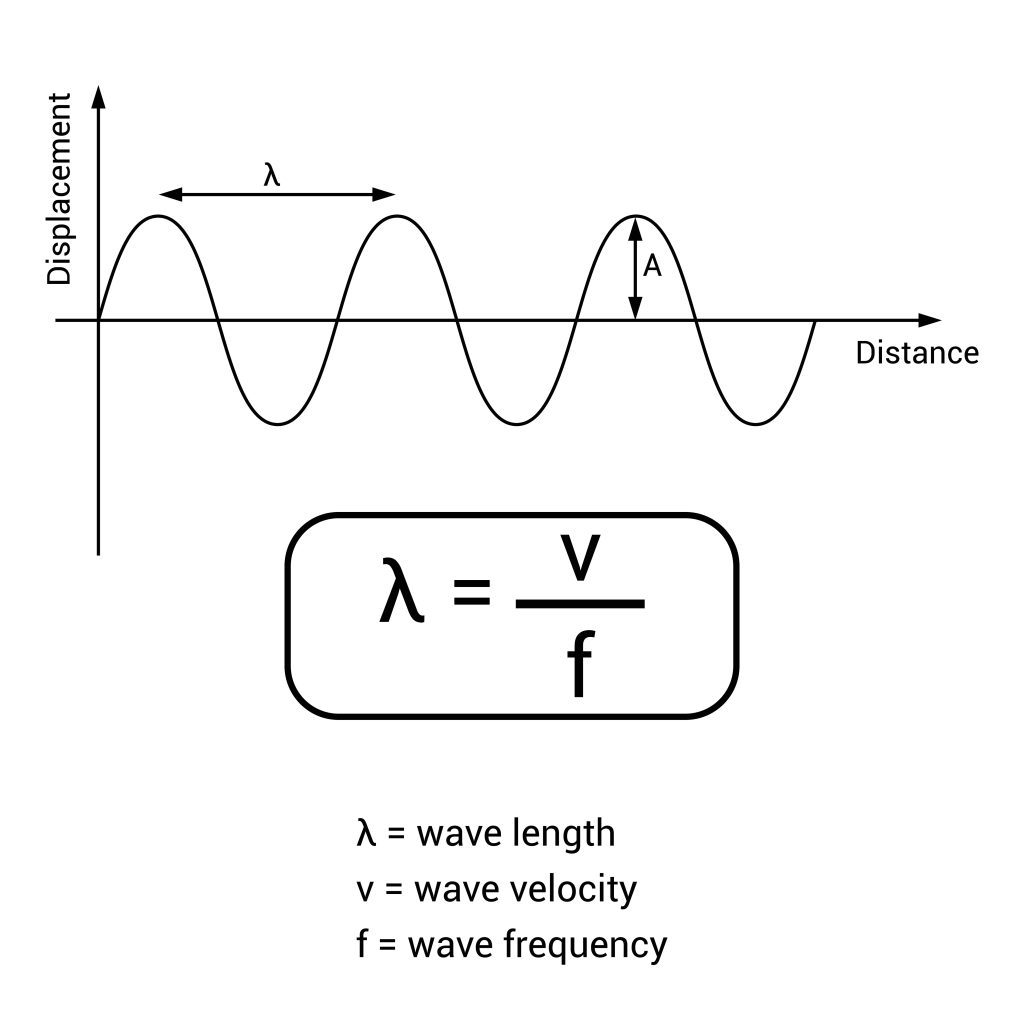
There are many different formulas used to calculate the length of wavelengths created by the movement of atomic electrons.
In the Rydberg formula, the wavelength is calculated by multiplying the hydrogen constant by the atomic number (i.e. hydrogen = 1) and multiplying that by a number that is calculated by subtracting the principal quantum number of the finished state from the initial quantum number of the initial state.
In this case, the answer, the wavelength of emitted light, is calculated as a fraction denoted by 1 over lambda, or, 1/ λ. The final formula looks like this:
1/ λ. = R * Zatomic number * (1/n22 – 1/n12)
Here, n2 is the final state while n1 is the starting state of the electron. This is the formula to compute the wavelength or energy level of any charge emitted by an electron.
How was the transition of electrons studied to discover wavelengths of light?
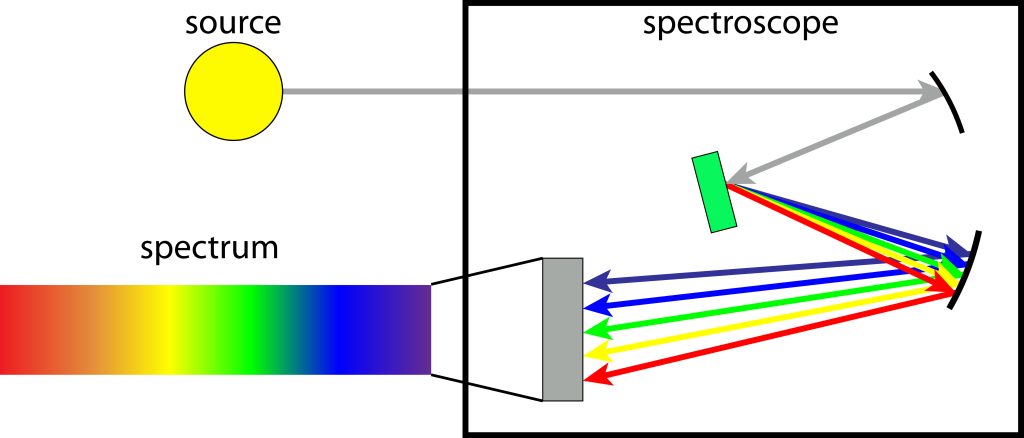
The transition of electrons was studied to discover wavelengths of light with the use of a tool called the spectroscope.
This was a tool invented specifically for the study of rainbows and was invented by Isaac Newton.
Then, it was called “modern spectroscopy,” but the study of electrons through tools like the spectroscope goes back to the 1600s and the good old days of Boyle and several of his very smart colleagues.
Newton was studying optics and prisms and wanted to understand the solar spectrum. He was the scientist who coined the word “spectrum” when he was trying to define what a rainbow was using light and wavelength language.
He was also cited as the scientist that taught us that when the colors of the rainbow were combined – the end result would be a white light.
Newton’s experiment included a prism-shaped tool that functioned somewhat like a camera on a tripod of sorts.
He would point it out the window to capture light rays and even rainbows. The light from the window would pass through the front of the tool and exit the back as white light.
Isaac Newton wanted to know why. He was not the first to create a tool such as this, but he was the first to really explain what happened when wavelengths transitioned into different particles.
Here, Newton would show us that the light changed color because the energy changed as the light passed through the spectroscope.
What does the spectroscope do?
Today, there are a number of spectroscopes used in quantum light theory, and they measure wavelengths of light and their transitions.
There is the emission spectroscope, the absorption spectroscope, and the reflection spectroscope.
The emission spectroscope measures photons and their energies when the energy is released.
Emission can be produced by a number of sources, including flames and sparks or a created electromagnetic wave.
Absorption spectroscopy is another form of spectroscope where photons are passed through a defined substance and the energy of the wavelengths is calculated.
A reflection spectroscope does what it sounds like, and measures wavelengths by looking at reflections.
When spectroscopy is used, it is used to study the transitions of light and its wavelengths.
This science began in the seventeenth century, before Isaac Newton, and examined prisms and optics.
Then, wavelength transition was not an exact science, but the Rydberg formula that arrived in 1888 would change that.
It is a formula that is still used today.
How do we know the transitions of wavelengths are accurate?
We know that the particle with the lowest principal quantum number is the number with the most energy.
The particle with the most energy is the one that needs to travel the least amount of distance. Today, it is calculated with the Rydberg formula and is considered exceptionally accurate.
An atom has a number of different energy levels that are frequently compared to a set of stairs.
Say a ball is an atom. When you throw the ball down a set of stairs, it is typically faster at the top of the stairs and tends to slow down at the bottom of the stairs.
This physics isn’t the same as quantum light physics, but the metaphor is similar in terms of the transition of light wavelengths.
The energy that has to travel the shortest will be the fastest.
There may still be light wavelengths at the bottom of the stairs, but here is where the notion of the light spectrum comes into play.
The wavelengths with the most energy will look closer to white light, while the colors will change as the ball, or the atom bounces down the stairs.
The Rydberg Formula is an adaptation of Balmer’s formula derived in 1885, which then did not include the atomic number in the formula.
Otherwise, the two formulas looked exactly the same.
To sum up, as with anything in quantum physics, there is no one answer as to what transition would give light the shortest wavelength.
The smaller its n, the shorter its wavelength. As Rydberg and so many other greats before him showed us, that number can always be anything.