Everything imaginable comprises this small matter called an atom. It is so small yet so vital to how everything operates.
An atom is the simplest form from which you can separate substances without the discharge of electricity.
Atoms comprise three parts. If you’re doing chemistry, you’ll learn that an atom is a foundation on which everything is created.
So, what are the three parts of an atom?
An atom comprises protons, neutrons, and electrons. Protons have a positive electrical charge, while neutrons do not.
Both are surrounded by electrons. These three types of atomic particles are not the only ones that you can find, as there are other subatomic components linked to them.
The majority of an atom’s mass resides in its nucleus, which is the positively charged core of the atom.
These three types of subatomic particles are not the only ones that can be found. However, they can only be made by adding massive quantities of energy, and they last only a few seconds.
Table of Contents
Is it true that most of an atom is empty?
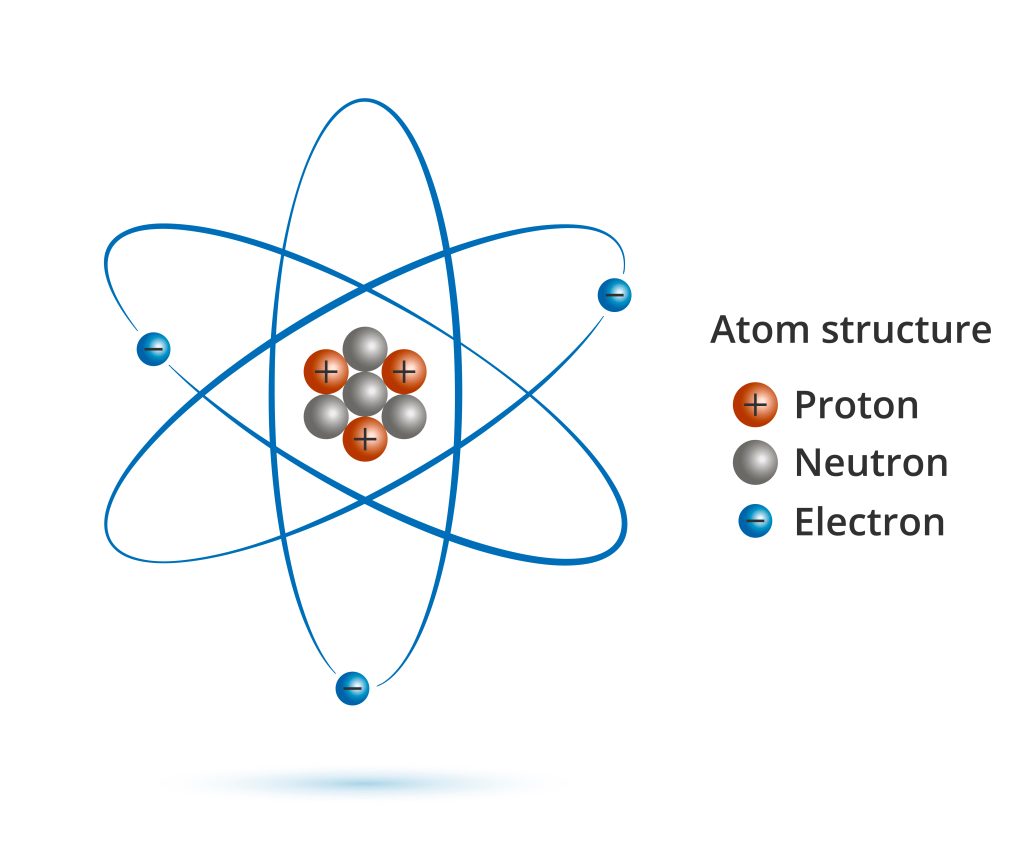
Space occupies the majority of an atom’s volume. Protons and neutrons form a positively charged nucleus, which is encircled by an electron cloud that is negatively charged.
The nucleus, which is the smallest and densest charged particle in existence, is contrasted to lightweight electrons.
A positive charge attracts electrons; in an atom, the electrons are held in place by their electric forces.
Can an image truly represent the characteristics of an atom?
For the atom, there is no single picture that can adequately convey its many qualities, and therefore, scientists must employ complementary images to illustrate different aspects of the atom.
You can compare the electrons in an atom to particles spinning around the nucleus in some regards.
There are some systems in which the electrons behave like waves that have been locked in place around the nucleus.
The dispersion of electrons is seen in these wave patterns, known as orbitals. An atom’s behavior and chemical properties are greatly impacted by these orbital features, and these orbital groupings, known as shells, are responsible for this.
What does most matter consist of?
Molecules make up the bulk of substance, and they can be separated quite readily. Atoms are bound together by chemical bonds that are hard to break into molecules, which themselves are made of atoms.

Electrons and nuclei are the smallest constituents of an atom.
These atoms are held together by the electric forces exerted by the charged particles themselves.
It takes ever-increasing quantities of energy and results in a wave of new subatomic particles, which are mostly charged, to divide these lesser component particles.
How big are atoms exactly?
Whether an atom has three electrons or ninety, they are all essentially the same size. A cubic centimeter of solid matter would contain around 50 million atoms (0.4 inches).
The angstrom (Å), which is equal to 10–10 meters, is a handy measure of length for quantifying atomic sizes.
One to two atoms have a radius of one to two micrometers.
How significant is the nucleus in terms of size?
The center is quite tiny when seen at about the atom’s entire size.
It occupies only 10–14 meters of the atom’s volume or one-hundredth of a percent of the atom’s volume.
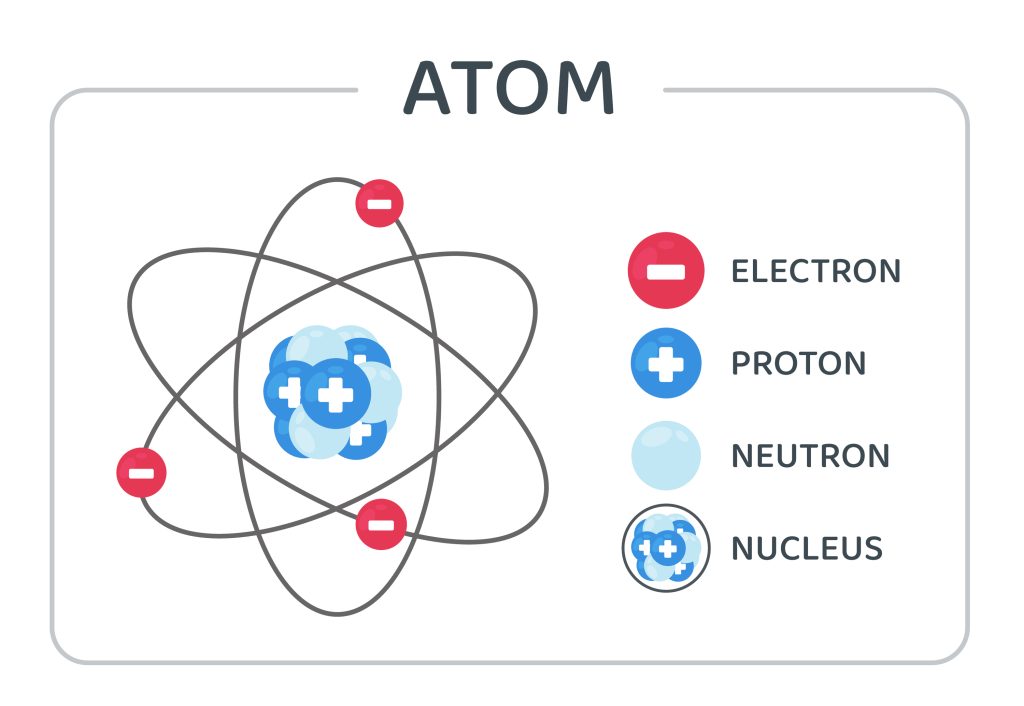
The femtometer (fm), which’s proportional to 10–15 meters, is a handy unit of length for determining nuclear sizes.
For a lighter nucleus like carbon, the diameter is about 4 fm, while for a huge nucleus like lead, it is 15 fm.
This range is relative to the number of particles that make up a nucleus. Despite its diminutive size, the nucleus contains nearly all of an atom’s mass.
How large are protons?
Positively charged protons are huge; neutrons, on the other hand, have no charge but are slightly heavier.
Having a wide range of protons and neutrons in a nucleus explains why their mass can vary so much.
When it comes to nuclei, heavier nuclei are almost half a million times bigger than the lightest element, hydrogen.
What is an atomic number?
The atomic number (indicated by the symbol Z), which would be the count of protons in the nucleus, is still the most essential feature of an atom.
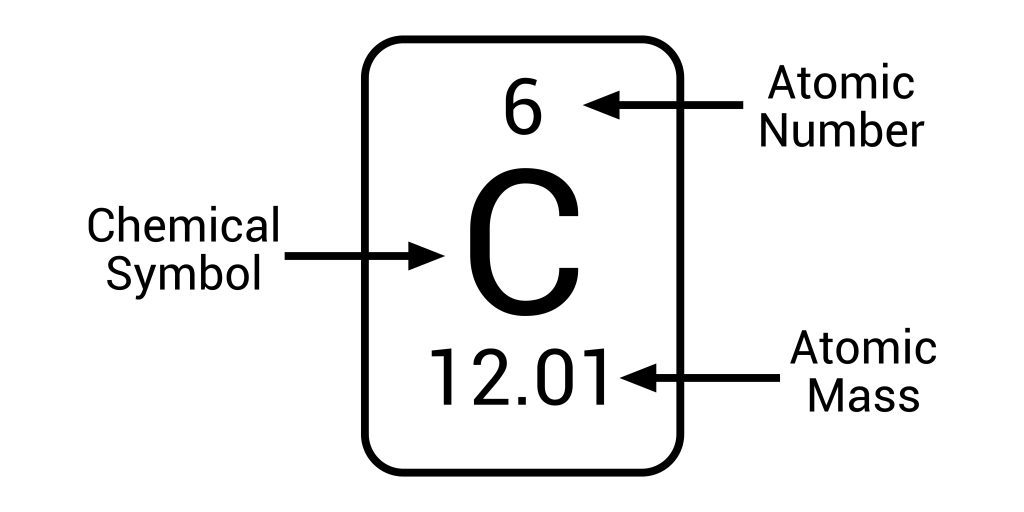
As an illustration, a Z of 6 indicates carbon, whereas a Z of 92 indicates the element uranium.
Neutral atoms have equal numbers of electrons and protons, just so the opposite charges are equal.
The proton numbers within the nucleus define an atom’s chemical characteristics because electrons govern how one atom reacts to another.
What are atomic masses and isotopes?
Neutrons in the nucleus do not affect the atom’s chemical characteristics, only its mass. Although the two masses are different, the chemical characteristics of a nucleus containing six protons as well as six neutrons are the same as a nucleus containing six protons plus eight neutrons.
Isotopes of one another are nuclei that have an equal number of protons and differ from neutrons.
There are several isotopes of every chemical element. According to the atomic mass number, the overall number of neutrons and protons in a nucleus can be characterized.
The atomic mass is defined as the sum of the nucleus’ mass and that of the electrons in one atomic mass unit.
As a result, the mass of a proton or neutron can’t be equal to the atomic mass unit.
What is the Millikan Oil-drop Experiment?
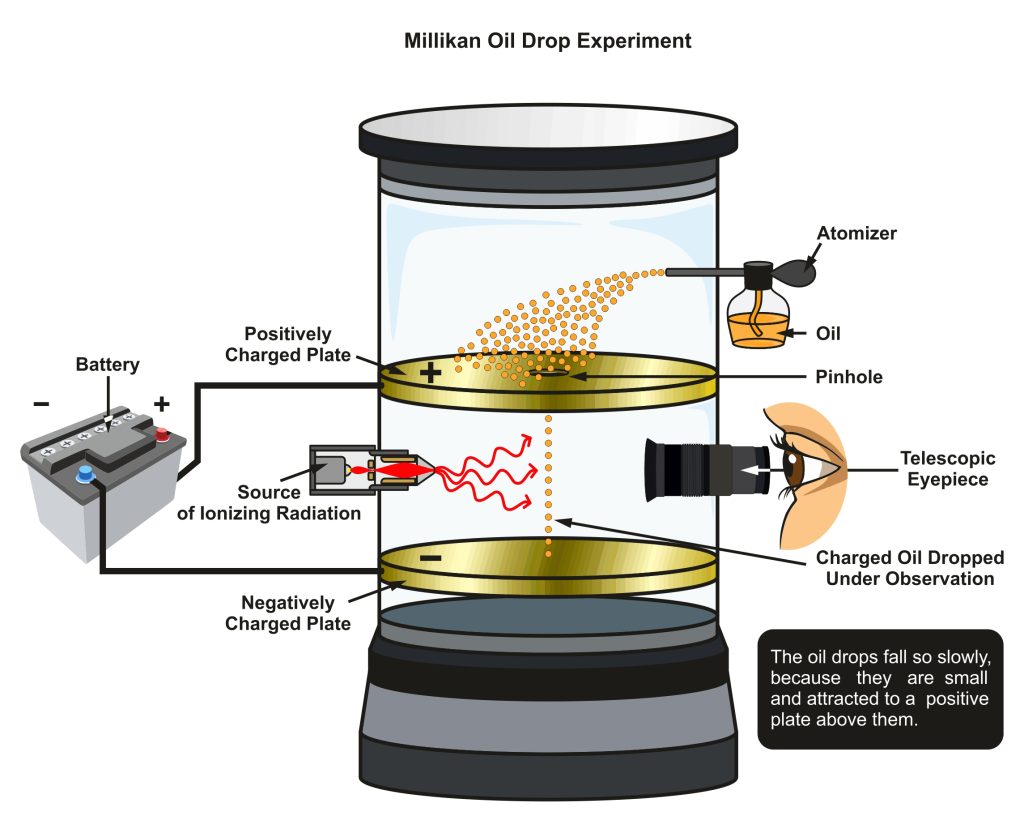
The discovery of the negative electric charge of an electron occurred during the late 1800s. From 1909 to 1910, Robert Millikan, an American physicist, became the first to calculate this charge’s level.
A container filled with oil mist was used by Millikan in his oil-drop test.
He was capable of determining the weight of oil droplets by observing how quickly they fell to the ground.
Electric forces can be used to slow or stop oil droplets that have accumulated some electrical charges as a result of friction, for instance.
Millikan was able to determine the charge on each drop by correlating the applied electric force with changes in motion.
After measuring a large number of drops, he discovered that all of the charges were multiples of the same single number.
Are charges on protons and electrons equal?
Robert Millikan looked at all of the droplets he was experimenting on and discovered that each one contained a simple multiple of the electron’s basic charge.
The proton’s charge is the same as the electron’s. However, the sign is reversed – the proton is positively charged.
Both of them are attracted to each other since they have opposite electric charges. Like gravity, this force is responsible for keeping electrons in orbit around the nucleus, much like it does for the Earth.
Do protons and neutrons have greater mass than an electron?
Approximately 10–28 grams x 9.10938291 is the weight of a single electron. The weight of a neutron or proton is approximately 1,836 times bigger is, which answers why the nucleus’ protons and neutrons are the primary determinants of an atom’s mass.
Electrons have other inherent characteristics. A type of this is known as spin. The electron can be compared to the earth, which revolves around its axis of rotation.
This characteristic can be found in the vast majority of subatomic particles.
They, on the other hand, reside in the subatomic realm and are therefore subject to the stipulations of quantum mechanics.
Because of this, these particles cannot be spun in any random direction, but only at a set rate at which they can.
Fermions are named after Enrico Fermi, an Italian American physicist who initially studied the physics of particles with half-integer spin during the first half of the twentieth century.
And in how electrons and protons and neutrons in the nucleus are ordered, fermions have a single important attribute.
Since no two fermions may inhabit the same configuration, the Pauli exemption principle (labeled after Wolfgang Pauli, an Austrian physicist) governs how electrons in an atom ought to have separate spin orientations to a moving electron the same as an electric charge.
Considering an electron to be a moving charge, we can think of them as small electromagnets.
A magnetic field causes an electron, like every magnet, to twist when it is present.
Think of the earth’s magnetic field like a compass needle facing north.
Electrons are said to have a moment of magnetism because of this characteristic. The magnetic moment in physics is a term used to describe the relationship between a magnetic field’s strength and the force it exerts on an item.
The magnetic moment of the electron measures 10–24 x -9.28 joule per tesla due to their inherent spin.
What are the electron’s energy and orbital levels?
Electrons, in contrast to planets spinning around the sun, may only exist in certain locations known as permitted orbits since they cannot exist at any random distance from the nucleus.
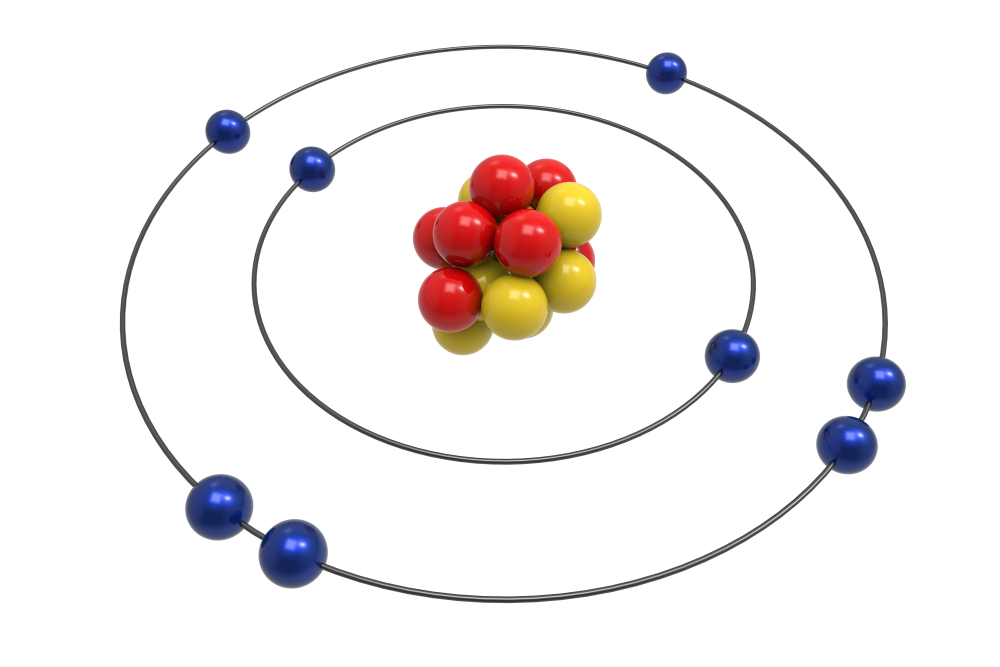
According to Niels Bohr’s 1913 work, quantum mechanics is responsible for this property; it states that the orbital momentum for an electron must appear in discrete assortments known as quanta, as does everything else.
An atom’s electrons can only be found in a limited number of orbits in the Bohr model, and these orbits have a range of energies.
An analogy for the orbits is a set of stairs with varying levels of gravitational potential energy, where a ball can be located on any step but never between them.